UNMET NEEDS

Pulmonary Arterial Hypertension affects more than 30,000 patients in the U.S. with an estimated five-year survival rate following diagnosis of 57%* (SEE FOOTNOTE)
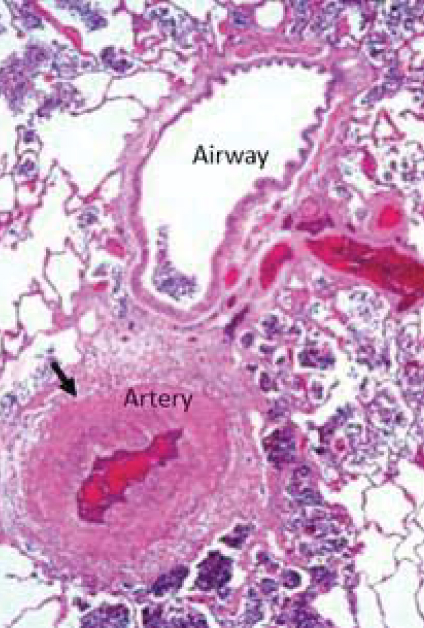
Pulmonary Arterial Hypertension (PAH)
WHO Group 1 PH
Pulmonary hypertension (PH) is a common complication of interstitial lung disease (ILD), a broad group of more than 200 lung disorders that cause scarring (fibrosis) of the lungs, with substantial morbidity and mortality.
In the U.S., an estimated 30,000 ILD patients also have increased pulmonary arterial pressures, which can adversely affect treatment outcomes and impact health care costs. The development of PH in ILD is associated with increased exertional oxygen requirements, diminished functional capacity, and decreased life expectancy.
*Based on data from the Registry to EValuate Early And Long-term PAH disease management (REVEAL) of patients in the U.S.
Learn more about our Phase 2B clinical trial in PAH [here]
Respira’s lead drug-device product candidate, RT234-PAH, is a first-in-class inhaled therapy intended for as-needed (PRN) use by patients experiencing symptoms. This contrasts with all other current PAH treatments, which are taken according to a chronic treatment regimen.